INDICATION
CUVITRU is a ready-to-use liquid medicine that is given under the skin (subcutaneously) to treat primary immunodeficiency (PI) in people 2 years and older.

Get to know your infusion supplies
You’ll become more familiar with them with each infusion.
Your doctor will order CUVITRU® [Immune Globulin Subcutaneous (Human)] 20% for you through a specialty pharmacy. Specialty pharmacies provide specific medication for certain chronic illnesses, like PI. Different than a regular pharmacy, specialty pharmacies have professionals who are specifically focused on—and trained for—filling prescriptions and providing the supplies necessary to help patients with these conditions.
The specialty pharmacy will send you what you need to infuse CUVITRU, including:
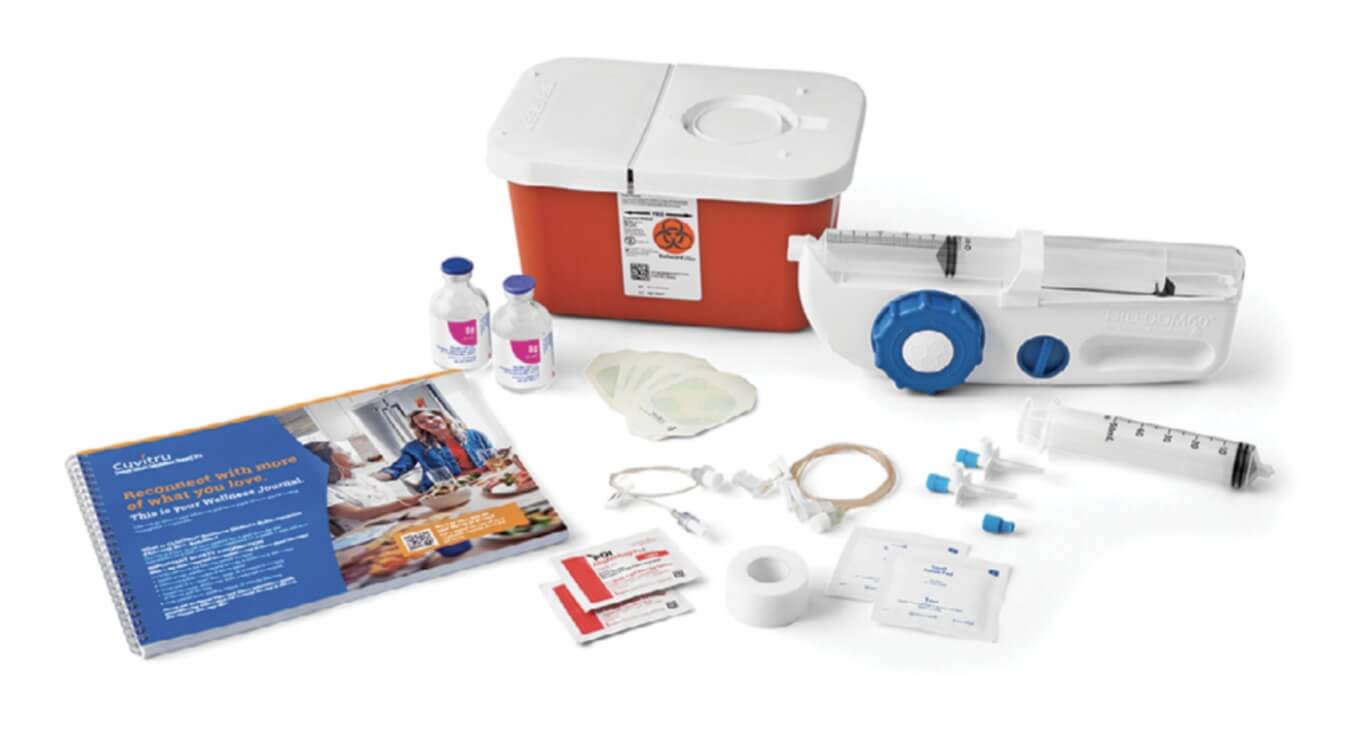
- CUVITRU vial
- Syringe(s)
- Sterile tip caps
- Infusion pump
- Transfer devices
- Subcutaneous needle set
- Alcohol swabs
- Tape
- Sterile clear bandage(s)
- Sharps container
- Gauze
This isn’t a complete list of all of the supplies you may need, but it is all of the basics. Also, there’s a CUVITRU starter kit, which includes a lot of great information to help you get educated and get started. It includes a Wellness Journal to record your treatments and jot down other notes, such as thoughts you have about infusing and questions for your doctor. Make sure you ask for your starter kit from your specialty pharmacy if you don’t get one.
When infusing, remember to refer to your Detailed Instructions for Administration for Patients or consult your healthcare provider. Takeda doesn’t prefer, recommend, or attest to using any specific infusion pump or other ancillary device.
It’s all about being prepared.
Get tips to make the most from your training with a nurse. It’s important that you don’t try infusing until you’ve trained with a nurse. Your nurse will show you how to safely and successfully self-infuse.
Want to know what infusing is like?
BEFORE the infusion
- Get comfy
- Get your supplies out and ready
- Read over the infusion steps as a refresher if you need to, especially if you’re just starting out
- If appropriate, make sure you’re hydrated before infusing and have a drink nearby in case you’re thirsty during your infusion
- If others are around, let them know your infusion time so there’s as little disruption for you as possible
DURING the infusion
- Try your best to relax and stay comfy, as the average infusion time is about 2 hours
- Read, play a game, catch up on your favorite show, call a friend—get creative, and use this time for you
- You may experience mild to moderate pain, redness, swelling, and itching (BUT these are common and generally go away within a few hours). These aren't all of the side effects, but they are ones you may immediately notice
- Other common side effects may include headache, nausea, fatigue, diarrhea, fever, and vomiting
- These are not all the possible side effects. Talk to your doctor about any side effect that bothers you or that does not go away. If side effects increase in severity or persist more than a few days, call your doctor or hospital emergency services immediately
- For additional safety information, click for Information for Patients
AFTER the infusion
- If appropriate, continue to drink fluids to stay hydrated
- Record your infusion details and any reactions or notes for yourself or your doctor

Wellness Journal
Keep your Wellness Journal handy to log your infusions and stay organized.
Here’s a closer look at the syringe driver pump.
To use your infusion pump, you insert the syringe filled with CUVITRU and then it slowly, steadily pushes your prescribed dose out of the syringe, through the tubing, to you.
- Syringe driver pump: pushes CUVITRU from the syringe
- Flow rate tubing: controls the infusion speed of CUVITRU
- Needle set: infuses CUVITRU into the subQ space
- Needle gauge: helps achieve the desired infusion rate of CUVITRU*
*To achieve the maximum infusion rate of 60 mL/hour/site with CUVITRU, you'll need a 24-gauge needle.
Takeda does not prefer, recommend, or attest to using any specific infusion pump or other ancillary device. The graphic is an example of what you can expect. Talk to your doctor or specialty pharmacist to ensure you’re receiving the supplies necessary to achieve your intended infusion rate.

Make room for relaxation.
It’s helped others to set up a dedicated space for infusing. Usually it’s a comfy, calm, and serene spot with a lounge-style chair, soft lighting to relax, and a few favorite things, like books, crossword puzzles, crafts, or games.
Remember the infusion process with

The infusion process is broken up into four sections. You may remember the “ABCs to infuse.” TRU4 replaces those! Why? Because we wanted to get more descriptive with the sections to make the process easier to remember—for you and everyone else who infuses.

Get organized
Gather supplies, check vials, and clean your work area.

Prep and draw
CUVITRU
Prepare the syringe, draw CUVITRU, and set up your pump tubing and needle set.

Start infusion
Select and clean your infusion site, insert and secure subcutaneous needle set, and infuse.

Remove needle(s)
and wrap up
You did it! Just a few “finish-up” details left—like cleaning up and making notes in your journal.
Sometimes it helps to see it for yourself. Totally get it.
The CUVITRU Infusion Video shows you how TRU4 all comes together during the infusion process.
- 0:00 Get ready to reconnect
- 2:12 Get organized
- 6:39 Prep and draw up medicine
- 10:12 Start infusion
- 12:42 Remove needles and wrap up