CUVITRU is indicated as replacement therapy for primary humoral immunodeficiency (PI) in adult and pediatric patients ≥2 years. CUVITRU is for subcutaneous use only.


Dosing and Administration
CUVITRU® can be infused at the fastest rates or highest volumes with the fewest needlesticks of any SCIG, without sacrificing tolerability1,2
SCIG=subcutaneous immune globulin.
Flexible administration
See how CUVITRU provides patients options.

Getting started with CUVITRU
To see important information for starting your patients on CUVITRU, explore the tabs below.
Explore administration combinations
Use the infusion calculator to determine CUVITRU administration parameter combinations for your patients based on needlesticks and frequency of infusions, and help your patients achieve CUVITRU’s maximum infusion rate by selecting the proper supplies.
Infusion calculator
Choose from the following parameters to find the right dosing plan for you and your patient.

Monthly
SCIG dose

Dosing
schedule

Infusion
time
| Needlesticks | 1 | 2 | 3 | 4 |
| CUVITRU |

Dosing
volume
per SCIG
20% infusion
The dosing calculator is meant to be used as a tool to help HCPs make informed decisions about dosing. Takeda is not making dosing recommendations.
CUVITRU: Maximum volume per site is 60 mL/site as tolerated (except for the first 2 infusions for patients <40 kg, for whom the maximum volume is 20 mL/site). Maximum infusion rate is 60mL/h/site as tolerated (after first 2 infusions). Up to 4 sites can be used simultaneously.1
What is the patient's weight?
What is the dose schedule?
Dose calculation of CUVITRU*
| Monthly dose | Dose per infusion |
| 300 mg/kg | |
| 400 mg/kg | |
| 500 mg/kg | |
| 600 mg/kg |
| Current monthly dose of IVIG/HyQvia | Dose calculation of CUVITRU† | |
|---|---|---|
| Monthly dose | Monthly dose | Dose per infusion |
| 300 mg/kg | 390 mg/kg | |
| 400 mg/kg | 520 mg/kg | |
| 500 mg/kg | 650 mg/kg | |
| 600 mg/kg | 780 mg/kg | |
For more information about HyQvia, please see Full Prescribing Information
*Monthly dose of CUVITRU is equivalent to current monthly dose of SCIG.
*Monthly dose of CUVITRU is calculated based on a dose adjustment factor of 1.30.
This calculator does not represent every possible weight calculation. Weights begin as 24 lb and increase in 4 lb increments to 288 lb.
See Full Prescribing Information for additional dosage recommendations. A conversion factor of 2.2046 lb/kg is used to convert the patient’s weight to kilograms. The values displayed are rounded to the nearest decimal point.
Select CUVITRU vial combinations based on dosage
CUVITRU is available in 1 g, 2 g, 4 g, 8 g, and 10 g vials of IG 20%. The dose must be rounded up or down, based on the physician’s clinical judgement.
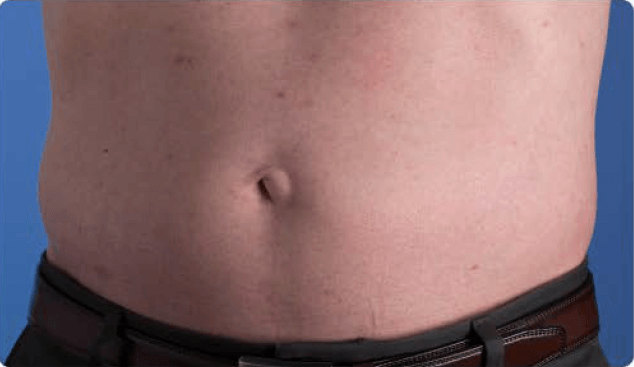
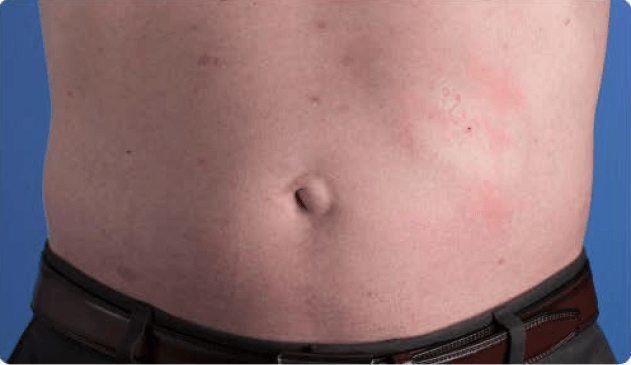
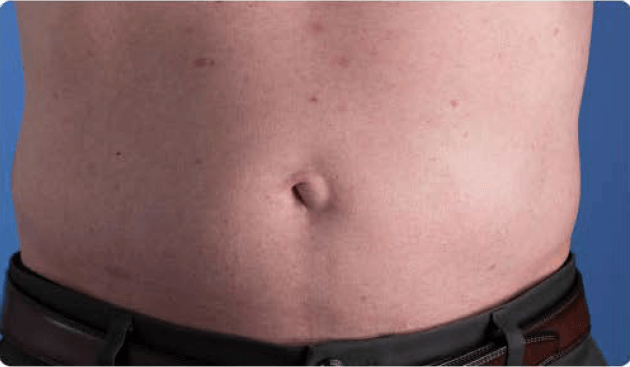
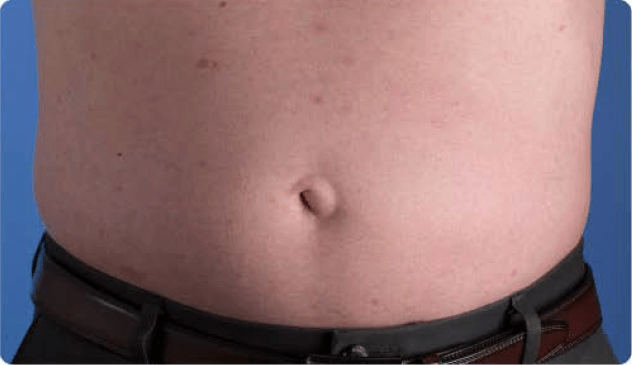
View examples of 2 patients’ infusion sites from pre-infusion to 24 hours after.
Volume infused per physician guidance based on patient’s specific clinical condition. It may be different for other patients.
Patient No. 1: Male, age 59
- 60 mL infusion in 1 site of the abdomen
- Infused at maximum rate of 60 mL/h/site
Patient No. 2: Female, age 14
- 40 mL infusion in 1 site of the thigh
- Infused at rate of 40 mL/h/site
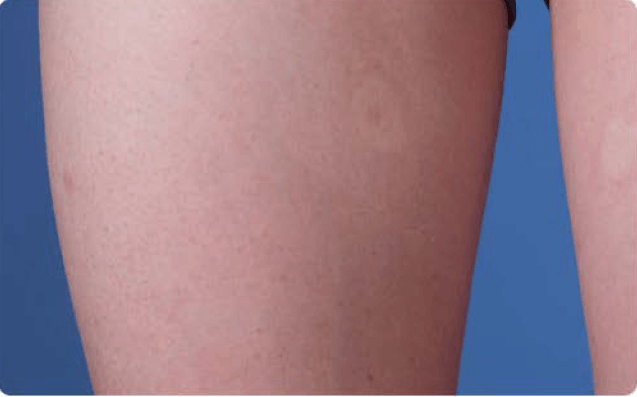
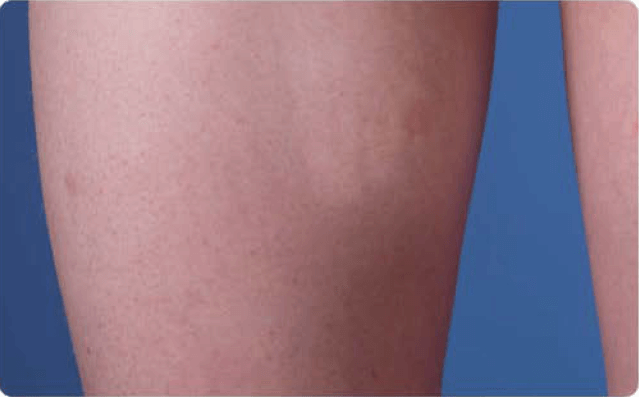
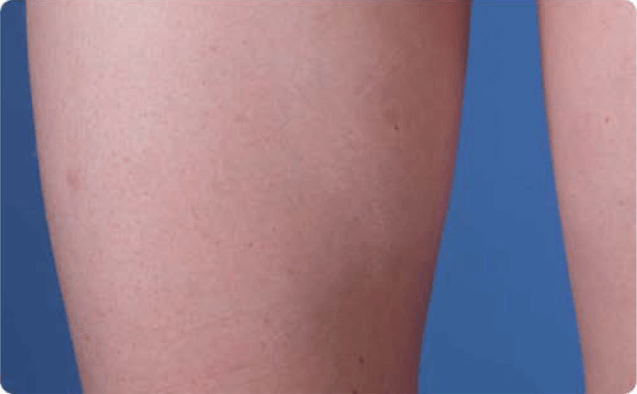
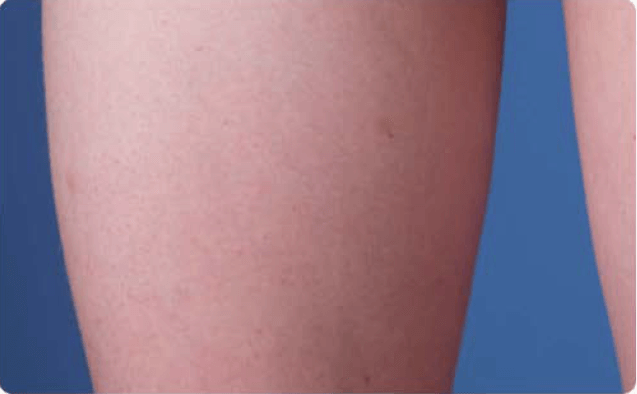
*1-hour infusion time.
Beginning patients on CUVITRU in a few steps
Learn about starting patients on CUVITRU
References
- CUVITRU. Prescribing information. Baxalta US Inc., 2021.
- Suez D, Stein M, Gupta S, et al. Efficacy, safety, and pharmacokinetics of a novel human immune globulin subcutaneous, 20% in patients with primary immunodeficiency diseases in North America. J Clin Immunol. 2016;36(7):700-712.
- Data on file. Takeda US Inc. 2015.